Back to Basics: What Is Soap and How Does It Work?
/Soap - it’s something that we all use everyday. We accept that it makes us cleaner than just plain water - but how? What IS soap anyway?
The FDA defines soap as “the alkalai salts of fatty acids”, which may or may not clear things up for you depending on how long ago your last chemistry class was.
“Soap” is what you get when you combine a fat or oil (fatty acids) and lye (an extremely strong “alkalai” or “base”). That’s all you need to make soap - fatty acid, lye, and a liquid (often water - though in The MacBath’s case, goat’s milk, which has more fatty acids of its own!)
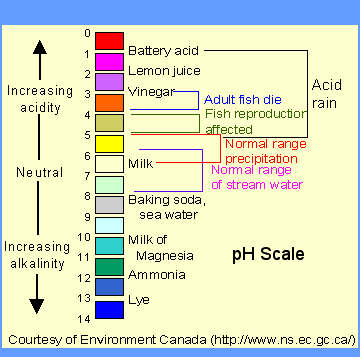
“Uh huh”. I hear you say. “I don’t see any salt whatsoever on that ingredient list. How can soap be a salt? I sprinkled it on my baked potato and it was awful!” First of all, stop tasting your soap. (I promise that no matter how good it smells it tastes terrible.) Second, ordinary table salt (sodium chloride or NaCL) is just one example of a “salt”. You might recall that, in chemistry, any combination of an acid and a base makes a salt. Soap is a combination of a weak acid (fatty acids) and a strong base (lye), which results in what is known as “alkalai salt,” or a salt that is basic on the pH scale. (See scale below) Sure enough, if you use a pH strip (also known as a litmus test) in soapy water, it often scores an 8 or 9.
“Whoa whoa whoa” I hear you cry. “I’ve been putting a base on my delicate skin all of these years? That sounds dangerous!” I promise you, you’re fine. “Acid” and “base” might sound like scary words, but you regularly consume weak acids and bases in your food every day. Soap is actually around the same pH as baking soda!
Ok, so I guess I’ve been washing all my life with "alkalai salts". How the heck does that get me clean?
Soap has two main mechanisms of action, both of which come from it being a surfactant, which comes from the words "surface active agent." The defining property of surfactants is the unusual ability to bind to both oil and water, which is the first and most important mechanism of action. Have you ever heard the phrase “they get along about as well as oil and water”? You know how you always have to shake up salad dressing before you pour it because it always separates into two layers? It’s because oil molecules and water molecules don’t like to mix! If you wash with plain water, it’s not getting all the oily goop off your body. That’s where soap comes in.
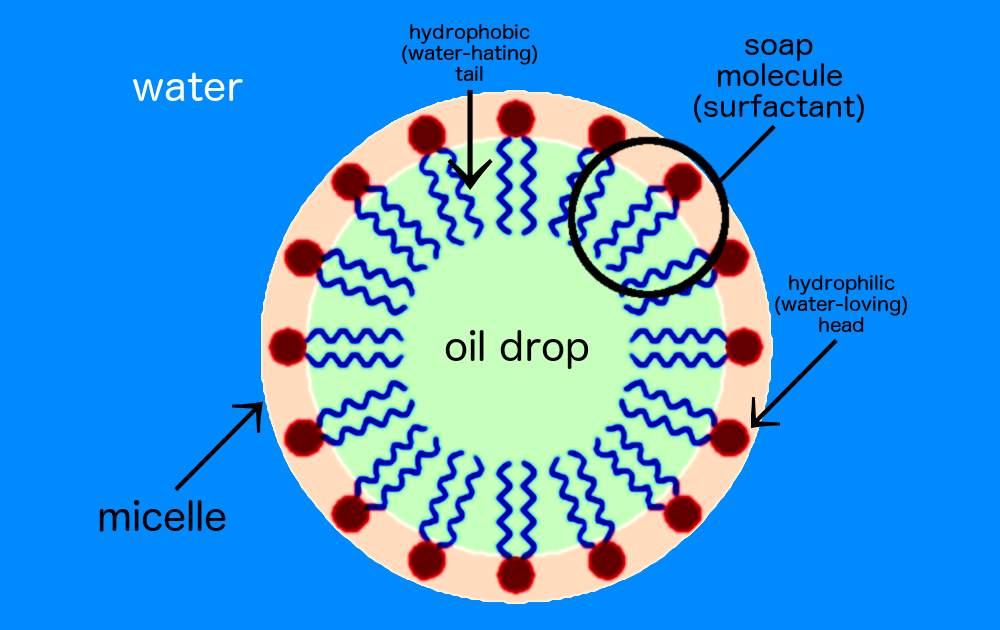
One end of the soap molecule is hydrophilic (water-loving) and binds to water (the black dots of the drawing below). The other end is hydrophobic (water-hating) hydrocarbon chain that binds to oil molecules (the purple tails in the drawing). This forms a cluster called a “micelle” — a ring of soap molecules surround the drop of oil, creating a bridge between oil and water. Thus, when you rinse off soapy water, it takes the oil with it. And ta-da! You’re clean.
A soap micelle.
The other mechanism of action is that it reduces the surface tension of water. Water molecules like to stick together — it’s why water beads up on your cold glass instead of spreading across it uniformly. Some people like to say that soap “makes water wetter” — what they actually mean is that by introducing soap molecules to water, the micelles force the water molecules spread out. If you’ve ever seen a water bug walking along the surface of a pond, this is because the water's surface tension can support the weight of the tiny bug. If you mixed soap into the pondwater (but, um, don’t), the water bug would slip right through the surface. The decrease in surface tension allows soapy water to get into more nooks and crannies than water alone.
If soap is just fats, lye, and liquid, then what is this “sodium lauryl sulfate” stuff I see on my soap packaging?
Ah, well, if you have sodium lauryl sulfate (abbreviated as SLS) in the ingredients list of your soap, bodywash, or shampoo, then The MacBath regrets to inform you that you do not actually have any soap—at least according to the FDA. What you’ve got is a synthetic detergent, which is also a surfactant — it has a hydrophobic and hydrophilic end — but it can react differently. If you’re not sure if what you have is a soap or a detergent, give it a test with a pH strip - detergents are neutral or acidic, whereas soap is always basic.
There are actually very few true soaps on the commercial market in the USA — nearly all the products available are synthetic detergents (yes, even your bar soap!). There are a two main reasons for this. One is that detergents are much cheaper to manufacture. Second, detergents perform differently than soap in extremely hard water — water heavy in minerals. If you have hard water that contains magnesium and/or calcium ions, the hydrophilic end of the soap molecule (Na+COO-) reacts with these ions to form soap scum. A detergent, whose molecule has a different salt at the hydrophilic end (Na+SO3-), won’t react with them the same way and won't produce soap scum. This must seem like a no brainer to commercial manufacturers — cheaper to produce AND works the same in any kind of water? Sign me up!
But — and you must have know there was a “but” coming — there are also downsides. Detergents are much more drying on the skin, and strip more of the body's natural oils. They’re also not as eco-friendly as soap — detergents take longer to break down and are harder on the environment when they get into the water table. Liquid detergents, such as shower gel and body wash, also require significantly more packaging than solid bar soaps, creating more waste in landfills So if your skin is dry or sensitive, or you’re trying to reduce your carbon footprint, you may want to give soap a try. Your skin and your planet will thank you.
Want more from us? Subscribe to our mailing list . And to sweeten the pot, when you sign up you get 10% off all MacBath goodies.